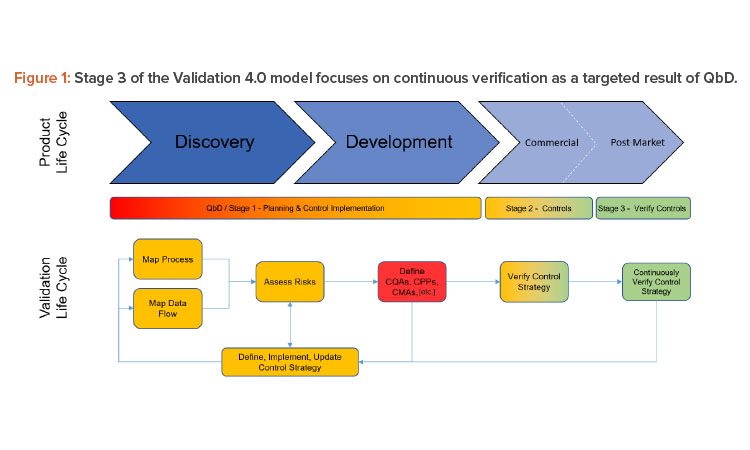
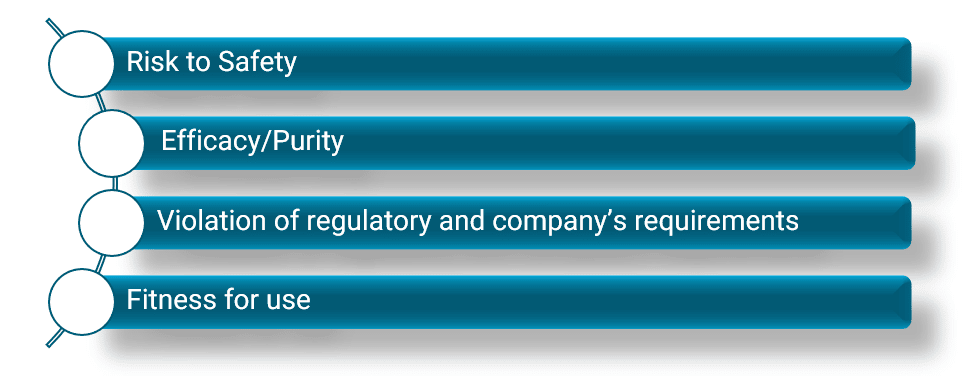
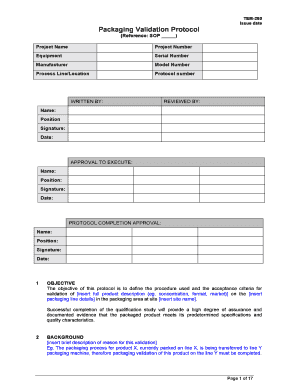
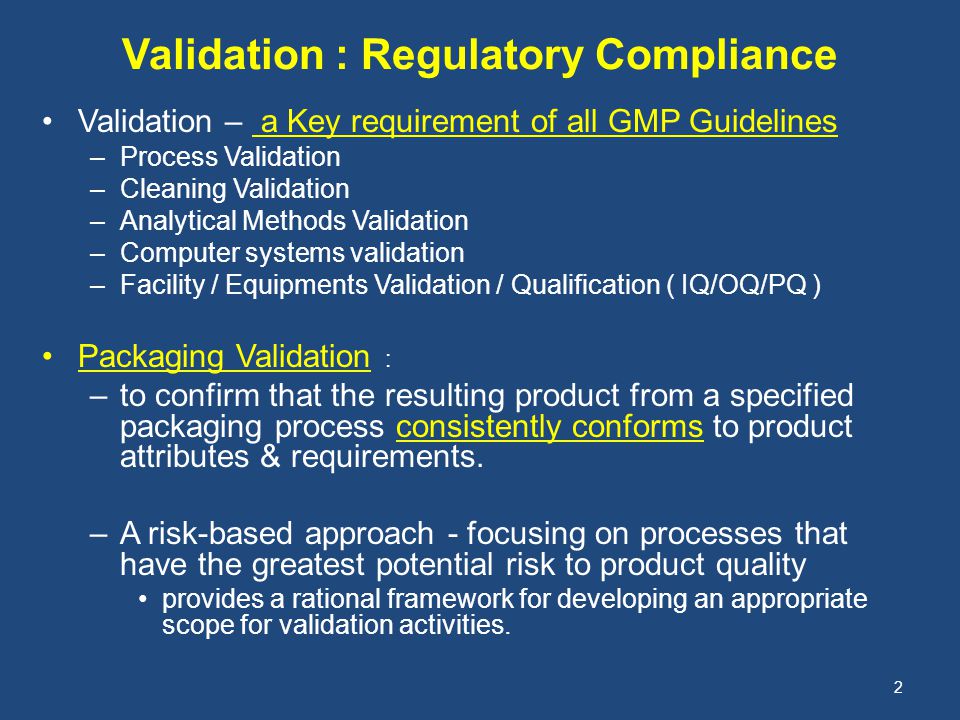
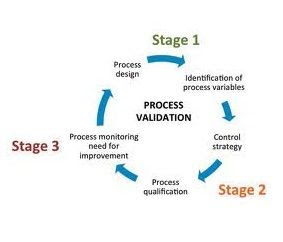
Overview of Packaging Validation for Drug Products | ISPE | International Society for Pharmaceutical Engineering

ISO 11607-2:2006, Packaging for terminally sterilized medical devices - Part 2: Validation requirements for forming, sealing and assembly processes: ISO/TC 198: Amazon.com: Books

Sustainability | Free Full-Text | A Systematic Literature Review on Packaging Sustainability: Contents, Opportunities, and Guidelines